
For Accredited Investors Only
INVEST IN A REVOLUTIONARY WAY
TO TREAT CANCER
Important Explainer Video
MAJOR MILESTONE UPDATE:
CancerVax Successfully Tricked Immune Cells
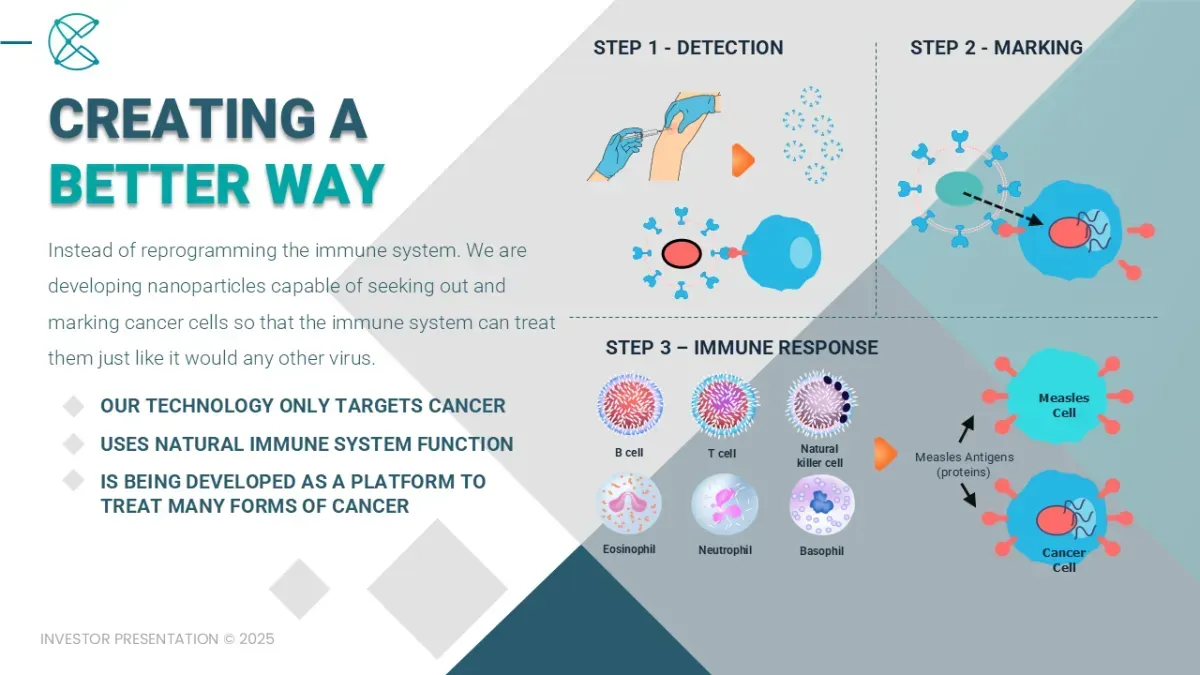
We are a Pre-Clinical Biotech Company developing a Universal Cancer Treatment Platform that will be customizable, as an injection, to treat many types of cancer.
Our innovative approach DETECTS, MARKS, and KILLS only cancer cells. By making cancer cells “look” like well-immunized common diseases such as measles or chickenpox, we intend to use the body’s natural immune system to easily kill cancer cells.
We look forward to the day when treating cancer will be as simple as getting a shot – A Revolutionary Way To Treat Cancer.
Become An Early Investor
Schedule an investment QA call
with an executive
OUR SCIENTIFIC TEAM
We have assembled a world-class team of experienced cancer scientists and advisors to help develop our revolutionary cancer technology.

George Katibah, PhD
Chief Scientific Officer

Adam Grant, PhD
Principal Scientist

Sumant Ramachandra, MD, PhD, MBA
Chief Scientific Advisor

Amit Indap, PhD
Scientific Advisor

Jonathan Lakey, PhD
Scientific Advisor

Matthew Spear, MD
Scientific Advisor

Steven Warner, PhD
Scientific Advisor
OUR DEVELOPMENT PARTNERS

Cytiva, formerly Precision NanoSystems, is a part of Danaher (NYSE: DHR) and a global leader in advancing and accelerating therapeutics. With a strong presence in life sciences research and bioprocessing, Cytiva provides innovative technologies and expertise to help companies bring life-changing treatments to market. The company specializes in biomanufacturing solutions, lipid nanoparticle (LNP) development, and cutting-edge analytical tools. CancerVax is working with Cytiva to leverage its clinically proven LNPs to to create a complete CancerVax therapeutic nanoparticle.

Axis Bio is a preclinical contract research organisation with specialist capabilities in oncology, inflammation and respiratory diseases. Services range from in vitro efficacy and mechanistic studies, to in vivo target engagement, with each study tailored to the unique requirements of the client. Our clients are spread across the globe and include pharmaceutical and biotech businesses, university-based research organisations and virtual/semi-virtual development companies. We listen, guide and advise clients through every step of the preclinical efficacy testing process, to deliver clear and robust results in a timely and cost-effective manner. This approach applies whether we are simply carrying out the in-life phase of a study or providing a complete package of analysis including flow cytometry analysis, bioanalysis and blood analysis, along with a detailed interpretation of results and recommendations.

TriLink BioTechnologies, a Maravai LifeSciences company, is a global leader in nucleic acid and mRNA solutions. TriLink delivers unrivaled chemical and biological experience, CDMO services, and high-quality readymade and custom materials, including its patented CleanCap® mRNA capping technology. Pharmaceutical leaders, biotech disruptors, and world governments depend on TriLink to meet their greatest challenges, from delivering the COVID-19 vaccine at warp speed to empowering innovative treatments in oncology, infectious diseases, cardiology, and neurological disorders to enabling future pandemic response plans.
FIGHT CANCER WITH BETTER TREATMENT

CancerVax Successfully tricked immune cells

PRESS RELEASES

DISCLAIMER:
The securities referred to in this website may be sold only to accredited investors, which for natural persons, are investors who meet certain minimum annual income or net worth thresholds. These securities are being offered in reliance on an exemption from the registration requirements of the Securities Act and are not required to comply with specific disclosure requirements that apply to registration under the Securities Act. The Securities and Exchange Commission has not passed on the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials. The securities are subject to legal restrictions on transfer and resale and investors should not assume that they will be able to resell their securities. Investing in securities involves risk, and investors should be able to bear the loss of their investment.
SAFE HARBOR STATEMENT:
Matters discussed in this website contain forward-looking statements. When used in this update, the words "anticipate," "believe," "estimate," "may," "intend," "expect" and similar expressions identify such forward-looking statements. Actual results, performance or achievements could differ materially from those contemplated, expressed or implied by the forward-looking statements contained herein. These forward-looking statements are based largely on the expectations of the Company and are subject to a number of risks and uncertainties. These include, but are not limited to, risks and uncertainties associated with our history of losses and our need to raise additional financing, the acceptance of our products and technology in the marketplace, our ability to demonstrate the commercial viability of our products and technology and our need to increase the size of our organization. Further information on the Company's risk factors is contained in the Company's quarterly and annual reports as filed with the Securities and Exchange Commission. The Company undertakes no obligation to revise or update publicly any forward-looking statements for any reason except as may be required under applicable law.
#CancerStories


